Hedinger, fundada en 1843, fabrica y distribuye Excipientes en la Industria Farmacéutica solo bajo condiciones GMP/GDP desde sus dos plantas localizadas en Alemania (Stuttgart, donde se encuentran las oficinas centrales, y Teutschenthal, cerca de Leipzig).
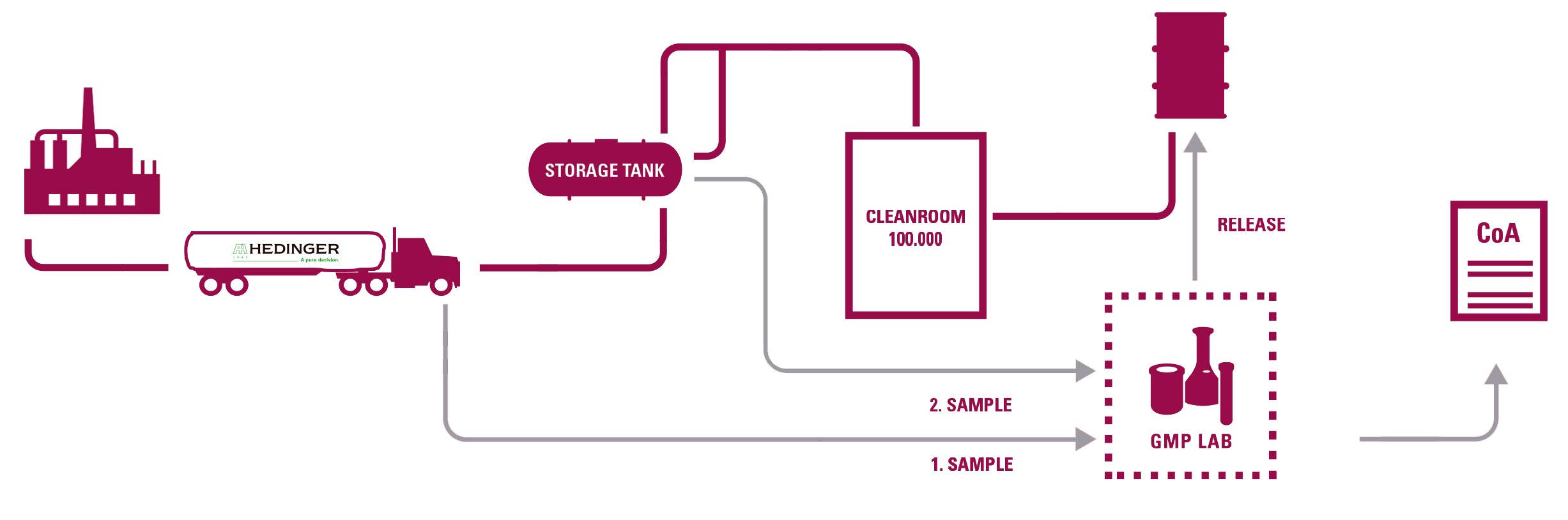
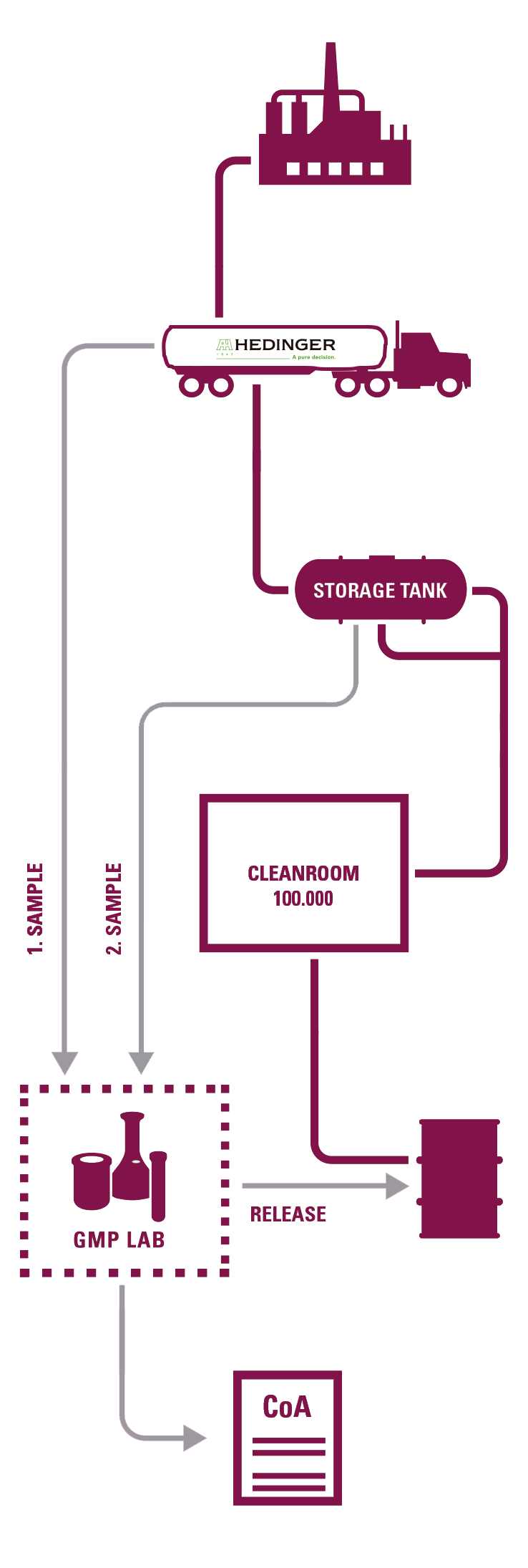
Ambas plantas están certificadas GMP por las autoridades locales alemanas y poseen 9 salas blancas en las cuales se re- envasan todos sus Excipientes Farmacéuticos.
Desde sus 2 laboratorios GMP pueden certificar de manera general todas sus operaciones acordes a las Farmacopeas Europea, Americana y Japonesa.
Gracias a sus instalaciones de última generación, en las que por política de calidad se reinvierte anualmente en su mejora, Hedinger puede ofrecer a los clientes que fabrican especialidades más críticas (biológicos, biosimilares, inyectables) excipientes con control microbiológico y de endotoxinas sometidos a un estricto control de calidad, así como el análisis específico de parámetros, adaptado a las necesidades del cliente. Prueba de ello es que los laboratorios fabricantes más reconocidos han depositado su confianza en la gestión de sus Excipientes.


Su catálogo de productos abarca tanto excipientes líquidos como sólidos, todos provenientes de fabricaciones globalmente reconocidos, sometidas a un proceso extra de control y / o purificación, que los diferencian de los grados técnicos y cosméticos normalmente encontrados en el mercado y por lo que son los de más alta calidad disponibles en la Industria Farmacéutica.
CUALIFICACIÓN DE PROVEEDORES
- Auditorías periódicas realizadas por responsables técnicos y científicos en sus instalaciones.
- Audit Reports de proveedores disponibles para consulta in situ por nuestros clientes.
ANÁLISIS Y LIBERACIÓN DE LOTES
- 2 Laboratorios GMP certificados por las autoridades competentes alemanas.
- Métodos implementados: Ph. Eur. / USP / JP.
- Robusto proceso de control e investigación de OOS.
- Cualificación de reference standards.
- Liberación de lote por la Persona Cualificada (QP), según la normativa GMP.
PRIMARY PACKAGING
- Control de entrada y liberación del envase primario y posibilidad de trazabilidad total.
- Sistema de etiquetado cualificado.
MUESTREO
- Documentación acorde a GMP (SOPs, records etc.) Retained samples de cada lote.
TRANSPORTE EN BULK DEDICADO
- Cualificación de tanques y equipo asociado y programa de entrenamiento de conductores.
RE-ENVASADO EN SALA BLANCA
- Cualificación acorde a EU GMP.
- Control rutinario de partículas y vigilancia microbiológica.
- Elevado número de personal específicamente entrenado por Hedinger (científicos, farmacéuticos, ingenieros).
REDUCCIÓN DE ANÁLISIS DE CONTROL DE ENTRADA PARA EL FABRICANTE DE MEDICAMENTOS
ESTUDIOS DE ESTABILIDAD
- Estudios de estabilidad específicos y personalizados.
- Reportes acordes a ICH Q1e.
- Condiciones medioambientales acorde a ICH (cuando sea necesario).
- Análisis In-house de parámetros relevantes.