Hedinger, founded in 1843, specializes in manufacturing and distributing excipients for the pharmaceutical industry exclusively under GMP/GDP conditions from its two plants in Germany—Stuttgart (headquarters) and Teutschenthal (near Leipzig).
Both facilities are GMP-certified by local German authorities and feature nine clean rooms dedicated to repackaging pharmaceutical excipients. Hedinger operates two GMP laboratories capable of certifying all operations according to European, American, and Japanese Pharmacopoeias.
Hedinger operates two GMP laboratories capable of certifying all operations according to European, American, and Japanese Pharmacopoeias.
With state-of-the-art facilities and a quality policy that mandates annual reinvestment in improvements, Hedinger provides excipients with microbiological and endotoxin controls for critical specialties such as biologics, biosimilars, and injectables. They offer tailored analysis of specific parameters to meet customer needs, a testament to the trust placed in Hedinger by leading manufacturing laboratories for their excipient management.


Their product catalog includes both liquid and solid excipients sourced from globally recognized manufacturers. These excipients undergo additional control and/or purification processes, distinguishing them from the technical and cosmetic grades commonly found in the market, ensuring they are of the highest quality available in the pharmaceutical industry.
SUPPLIER QUALIFICATION
- Periodic audits conducted by technical and scientific personnel at their facilities.
- Supplier Audit Reports available for on-site consultation by our customers.
BATCH ANALYSIS AND RELEASE
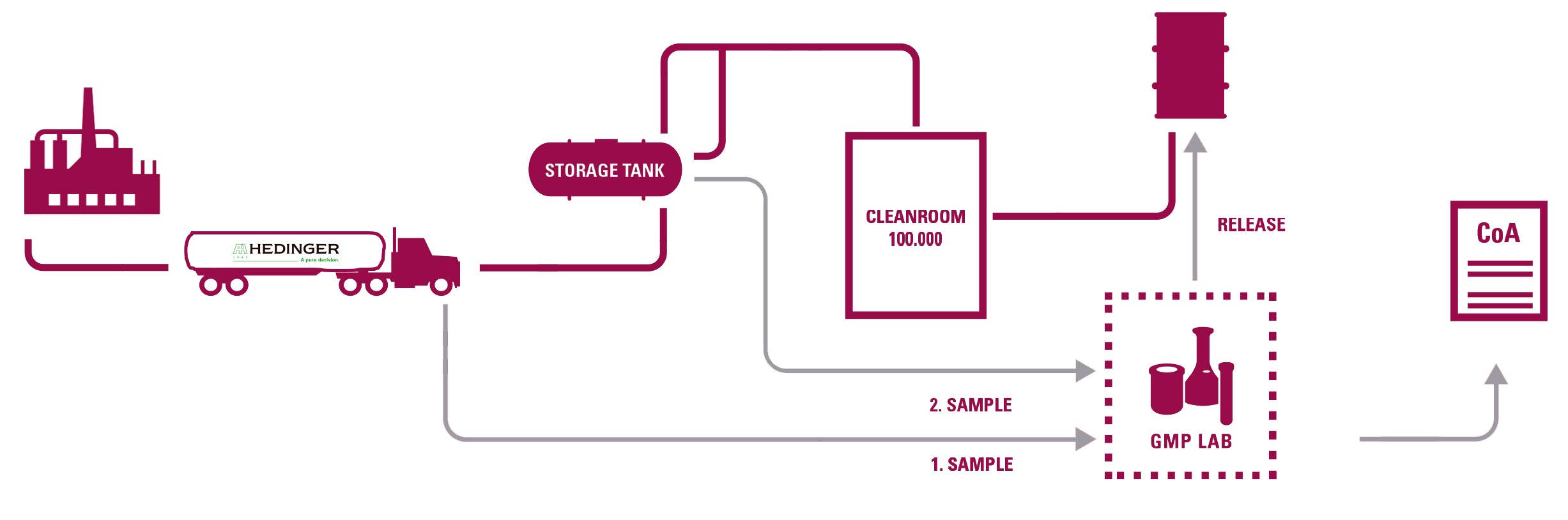
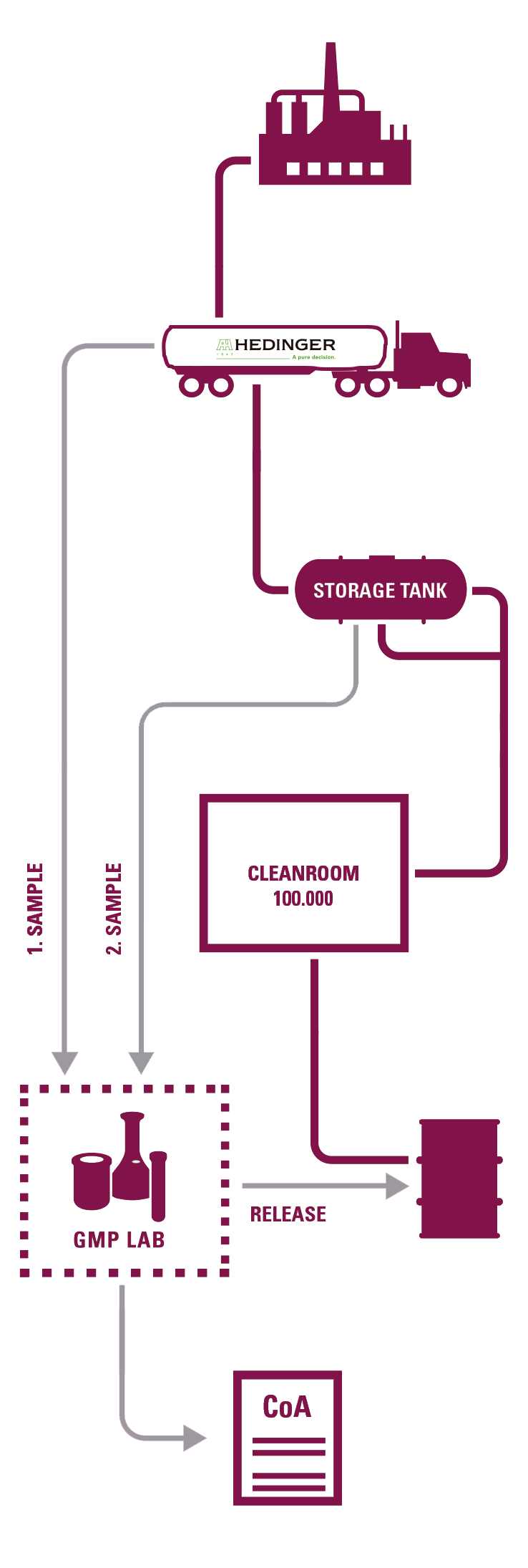
- 2 GMP-certified laboratories by the competent German authorities.
-Implemented methods: Ph. Eur. / USP / JP.
- Robust OOS control and investigation process.
- Qualification of reference standards.
- Batch release by the Qualified Person (QP) as per GMP regulations.
PRIMARY PACKAGING
- Incoming control and release of primary packaging with the possibility of full traceability.
- Qualified labeling system.
SAMPLING
-Documentation in accordance with GMP (SOPs, records, etc.). Retained samples of each batch.
DEDICATED BULK TRANSPORTATION
- Qualification of tanks and associated equipment and driver training program.
REPACKAGING IN CLEAN ROOM
-Qualification according to EU GMP.
- Routine particle control and microbiological monitoring.
- A large number of specifically trained personnel by Hedinger (scientists, pharmacists, engineers).
Streamlining Incoming Quality Control for Pharmaceutical Manufacturers
STABILITY STUDIES
- Specific and customized stability studies.
- Reports in accordance with ICH Q1e.
-Environmental conditions according to ICH (when necessary).
- In-house analysis of relevant parameters.