EXCIPIENTS
Synthetic Glycerin
Its manufacturing process guarantees the obtainment of a highly pure and stable product that complies with the highest regulatory standards of the Pharmaceutical Industry.
Origin of Synthetic Glycerin
Synthetic Glycerin, produced by Blue Cube in their Stade plant (Germany) is distributed by our Hedinger representative from their two plants located in Germany.
Hedinger, founded in 1843, makes and distributes excipients for the Pharmaceutical Industry under GMP/GDP conditions.
Thanks to their state of the art facilities, they offer laboratories that make more critical specialities (biologists, biosimilars, injectables as well as ophthalmics) Glycerin with microbiological and endotoxins subjected to a strict quality control (parenteral grade quality). For the less critical specialties (syrups, creams, gels, vaginal applicators, suppositories, ointments, child related specialities, etc…) they offer Pharma quality in the same GMP compliance.

MANUFACTURING PROCESS AND TRANSPORT SUBJECTED TO THE STRICTEST QUALITY CONTROL

PURITY
- It doesn’t contain allergens
- It doesn’t contain aflatoxins
- It doesn’t contain pesticide residues
- In agreement with the ICH Guideline for residual solvents
STABILITY
- Extremely low level of aldehydes. Certified by qualified personnel
- Odorless and colorless throughout the shelflife of the product
- Excellent shelflife. 36 months vs. 12/24 months for plant based Glycerin.
CERTIFICATES
- Excipact
- GMP (German Authorities)
- ISO 9001, ISO 14001
- OSHAS
REGULATORY QUALITY SUPPORT
- Regulatory GMP Quality questionnaires
- Audits
- Technical Package
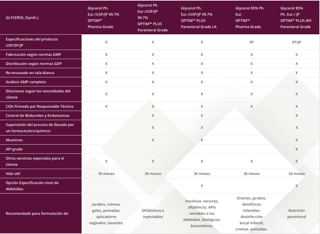
| GLYCEROL (Synth.) | Glycerol Ph. Eur./USP/JP 99.7% OPTIM™ Pharma Grade |
Glycerol Ph. Eur./USP/JP 99.7% OPTIM™ PLUS Parenteral Grade |
Glycerol Ph. Eur./USP/JP 99.7% OPTIM™ PLUS Parenteral Grade LA |
Glycerol 85% Ph Eur. OPTIM™ Pharma Grade |
Glycerol 85% Ph. Eur./ JP OPTIM™ PLUS API Parenteral Grade |
| Especificaciones del producto USP/EP/JP | X | X | X | EP | EP/JP |
| Fabricación según normas GMP | X | X | X | X | X |
| Distribución según normas GDP | X | X | X | X | X |
| Re-envasado en sala blanca | X | X | X | X | X |
| Análisis GMP completo | X | X | X | X | X |
| Diluciones según las necesidades del cliente | X | X | X | X | X |
| COA Firmado por Responsable Técnico | X | X | X | X | X |
| Control de Bioburden y Endotoxinas | X | X | X | ||
| Supervisión del proceso de llenado por un farmacéutico/químico | X | X | X | ||
| Muestreo | X | X | X | ||
| API grade | X | ||||
| Otros servicios especiales para el cliente | X | X | X | X | X |
| Vida útil | 36 meses | 36 meses | 36 meses | 36 meses | 24 meses |
| Opción Especificación nivel de Aldehídos | X | X | |||
| Recomendado para formulación de: | Jarabes, cremas, geles, pomadas, aplicadores vaginales, laxantes. | Oftálmicos e Inyectables | Insulinas, vacunas, oftálmicos, APIs sensibles a los aldehídos, biológicos, biosimilares. | Enzinas, jarabes, dentífricos infantiles, desinfección bucal infantil, cremas, pomadas. | Nutrición parenteral |
Trust in Globalk Química
Download your Glycerin catalogue.



